To decrease the incidence of chemotherapy-induced myelosuppression in patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen
For extensive-stage small cell lung cancer (ES-SCLC)
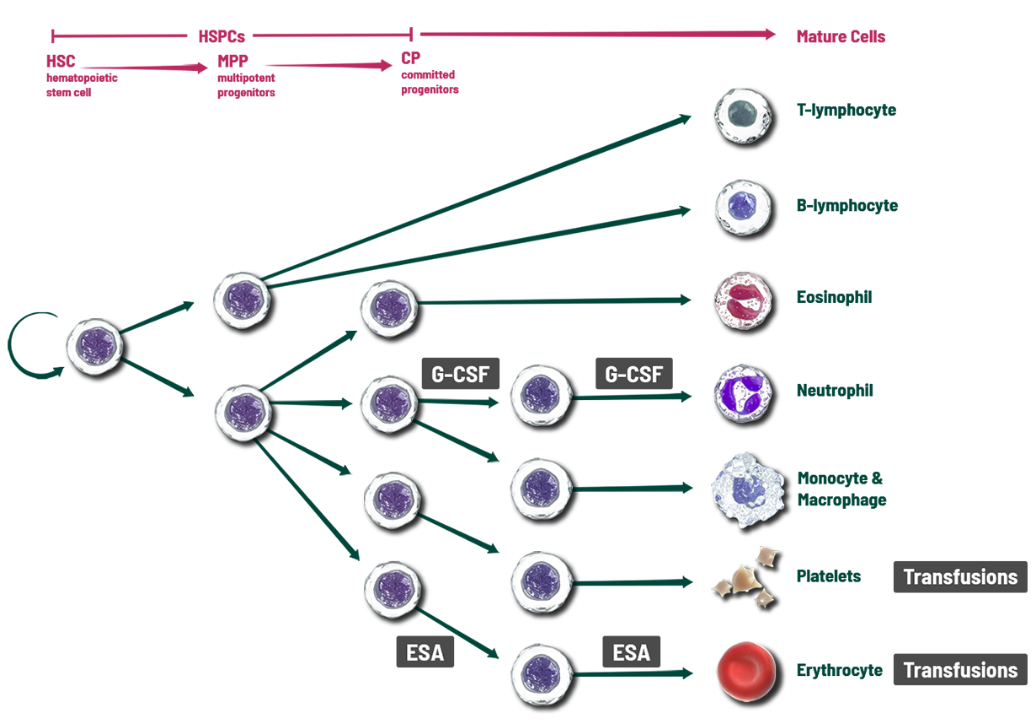
MYELOPROTECTION: PROACTIVELY PROTECT THE SOURCE OF NEUTROPHILS, RBCs, AND PLATELETS
Only COSELA Provides Myeloprotection Before Chemotherapy Damage
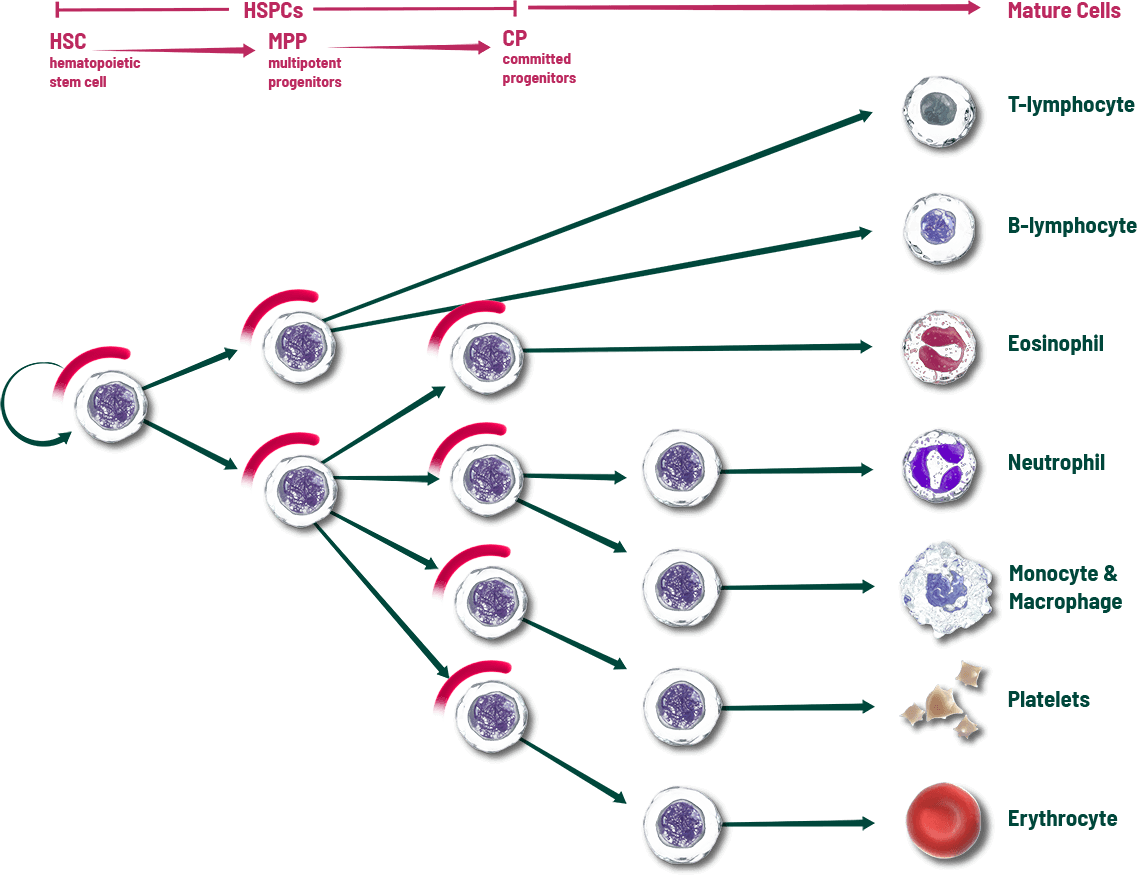
When COSELA® (trilaciclib) is given prior to chemotherapy, it transiently arrests hematopoietic stem and progenitor cells (HSPCs), the source of blood cell lineages, in the G1 phase of the cell cycle to help protect them during chemotherapy.
- One Therapy, Multiple Lineages
Protect the source of your patient’s neutrophils, RBCs, and platelets. - Protects Before Chemotherapy Damage
COSELA is not a growth factor; growth factors and transfusions address individual cell lines after the effects of chemotherapy. - Protect Patients’ Bone Marrow
With each chemotherapy cycle, added damage can occur to a patient’s bone marrow. COSELA can protect starting with the 1st cycle and throughout the treatment course.
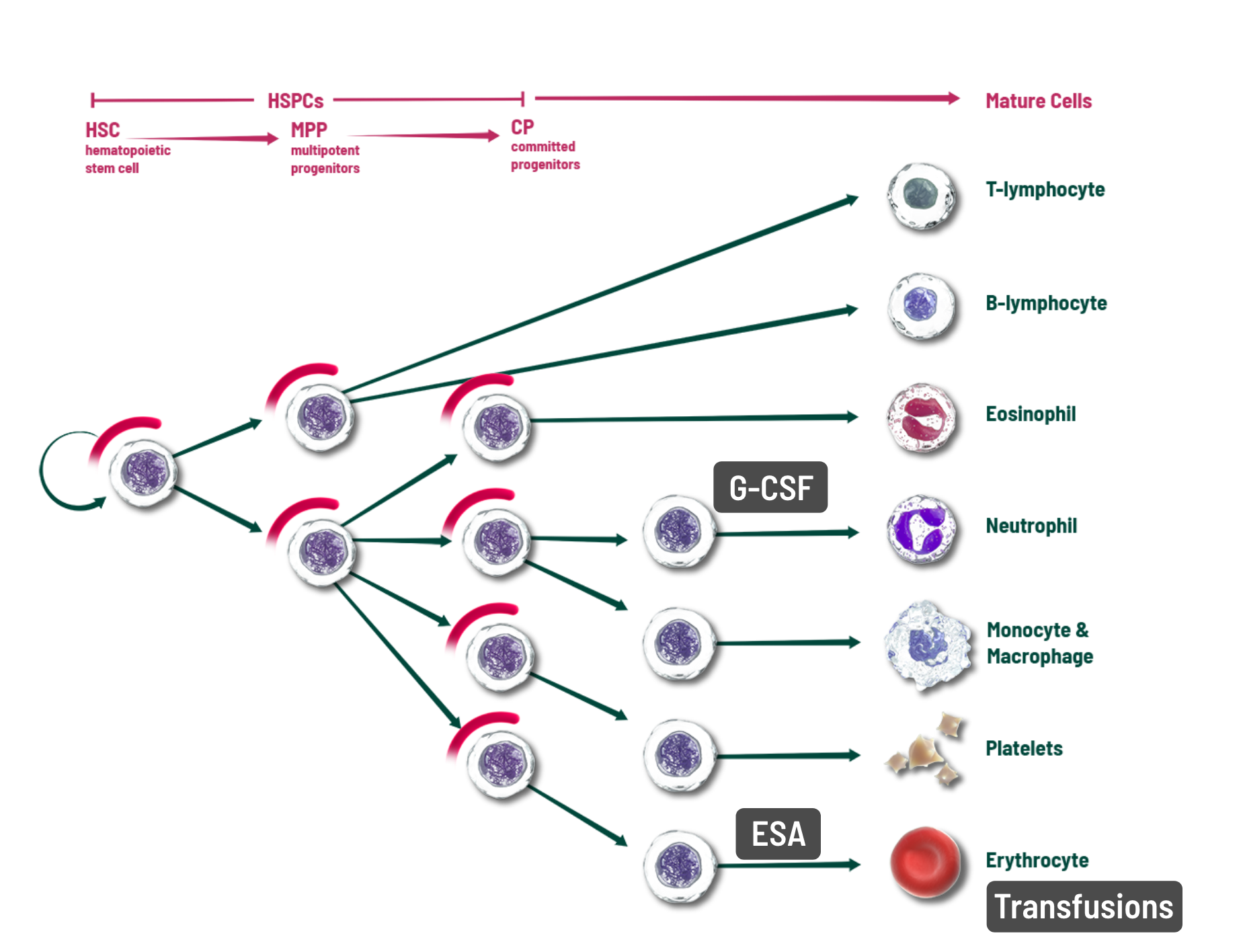
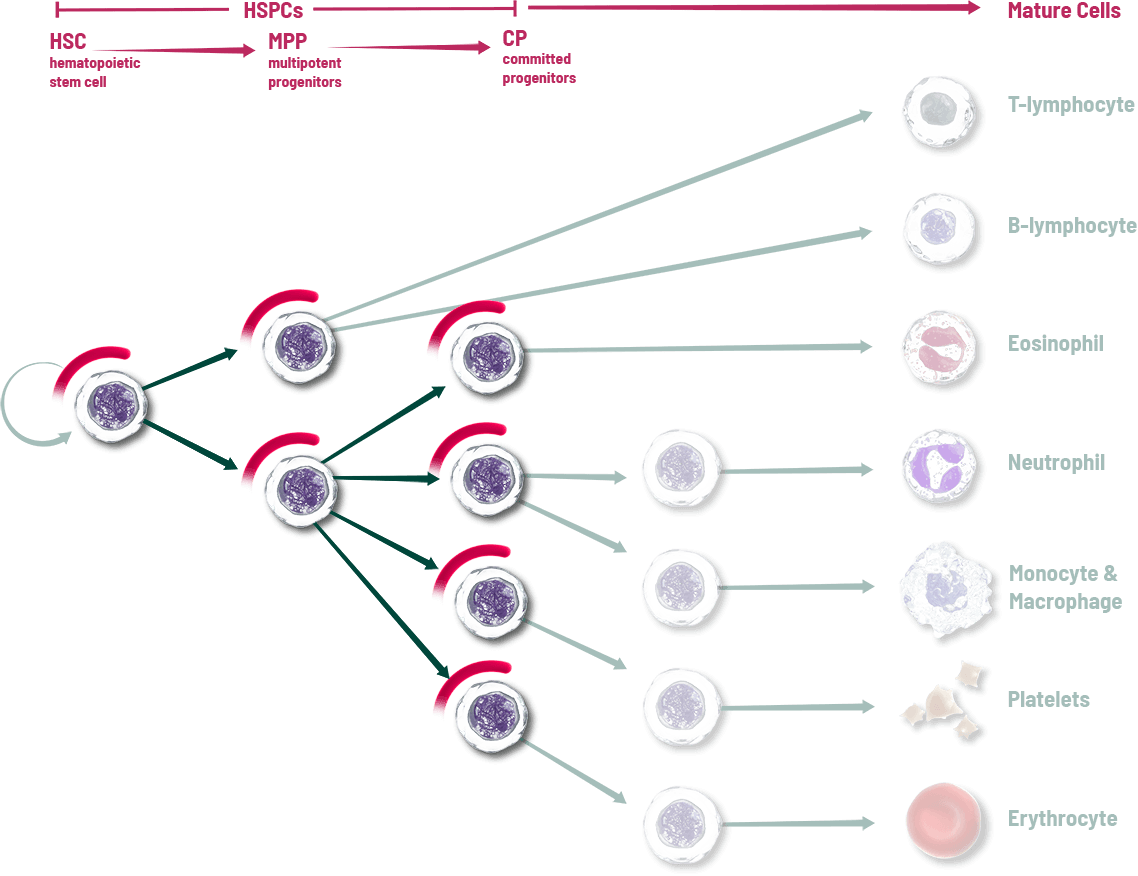
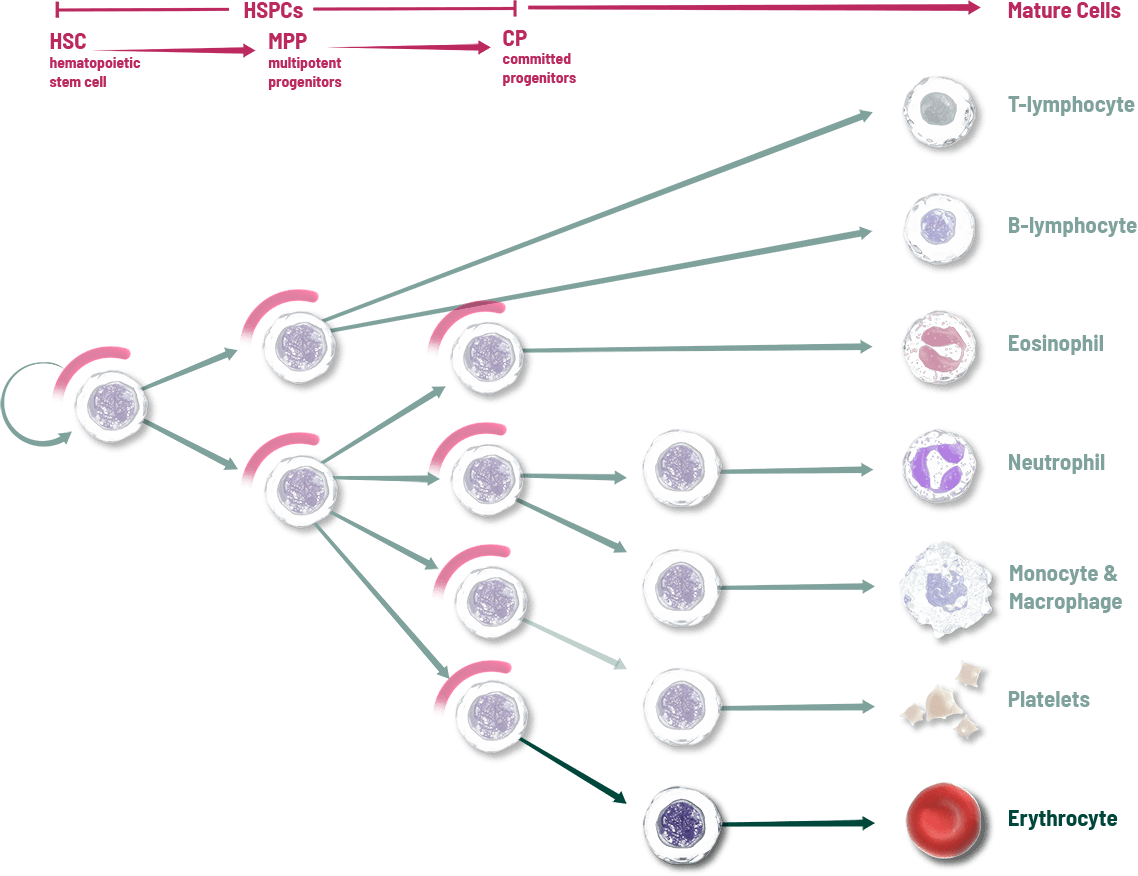
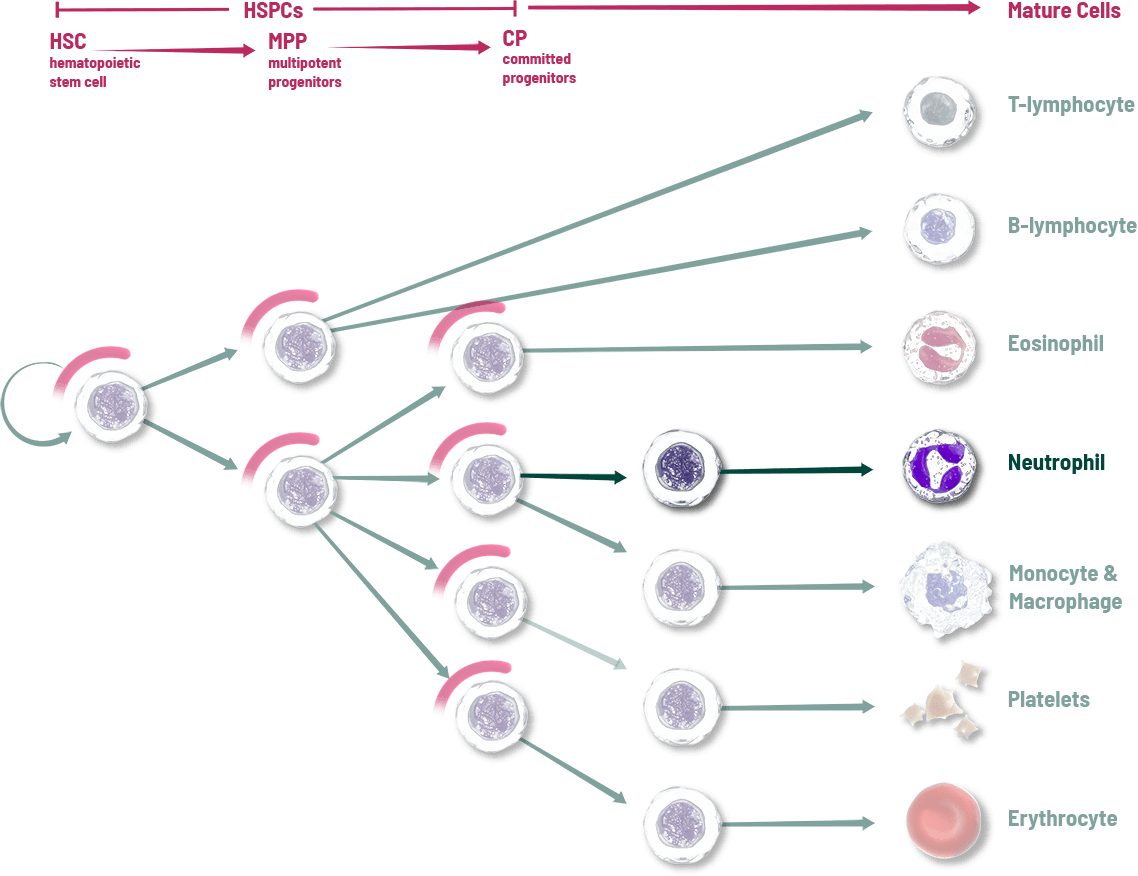
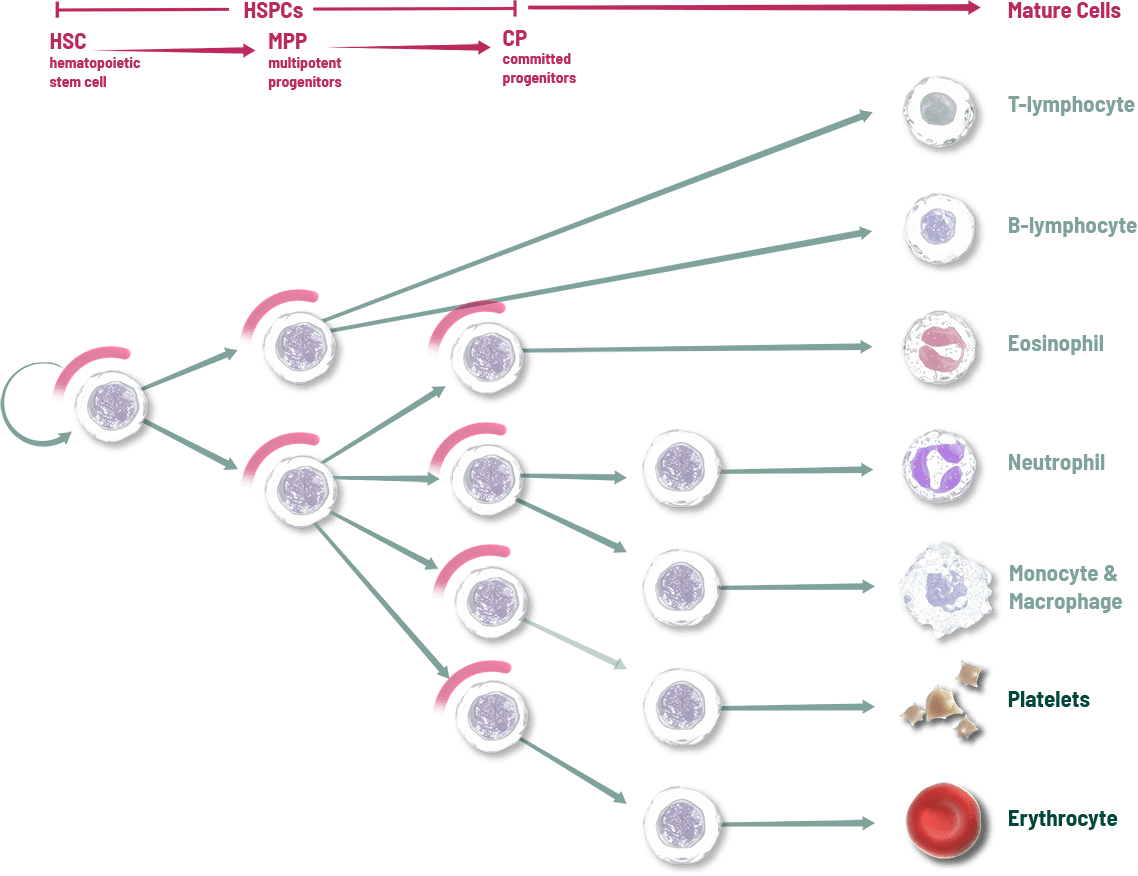
Other interventions attempt to address individual cell lines after the effects of chemotherapy1
- Myeloprotection strategy: When given prior to chemotherapy, COSELA transiently arrests HSPCs in the G1 phase of the cell cycle
WHEN COSELA IS GIVEN intravenously PRIOR TO CHEMOTHERAPY, IT has a unique effect on the HSPC cell cycle to HELP provide protection
COSELA® (trilaciclib) provides transient inhibition of the CDK4/6 pathway when given prior to chemotherapy (on days chemotherapy is administered)
- Proliferating HSPCs are susceptible to chemotherapy damage, resulting in myelosuppression; their proliferation is dependent on cyclin-dependent kinase (CDK) 4 and 6 activity
- COSELA transiently arrests HSPCs in the G1 phase of the cell cycle
-
This temporary arrest has been shown to help protect HSPCs from chemotherapy-induced damage
- After removal of COSELA, HSPCs are able to resume activity to help maintain bone marrow function2*
*Effect demonstrated in in vivo preclinical studies.
INDICATION: COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC).
WATCH AND INTERACT WITH THE COSELA MOA VIDEO
WATCH THE COSELA MOA VIDEO
COSELA helps protect hematopoietic stem and progenitor cells (HSPCs), the source of multiple blood cell lineages. Explore the interactive blood cell lineage at the end of the video that shows the impact of COSELA and other interventions.
COSELA helps protect hematopoietic stem and progenitor cells (HSPCs), the source of multiple blood cell lineages. See the mechanism of action (MOA) of COSELA.

Myeloprotection: A Proactive Approach to Help Protect Against Myelosuppression Caused by Chemotherapy
Hematopoietic stem and progenitor cells (HSPCs) are a critical factor for patients with extensive-stage small cell lung cancer receiving chemotherapy.
These key cells in the bone marrow are the source of blood cell lineages including neutrophils, red blood cells, and platelets. Unfortunately, these rapidly dividing HSPCs are vulnerable to damage when chemotherapy is used to kill tumor cells, which can result in the depletion of multiple blood cell lineages and a range of adverse events including neutropenia, anemia, and thrombocytopenia.
These adverse events can have significant clinical consequences, including risk of infection, fatigue, reduced quality of life, and the need for interventions.
To address the effects that chemotherapy has on HSPCs and blood cell lineages, growth factors can be used to stimulate surviving committed progenitor cells after the effects of chemotherapy and transfusions can be given to replace red blood cells and platelets.
COSELA is the first and only therapy that provides a novel approach to the clinical challenge of myelosuppression by proactively helping to protect HSPCs, to enable myeloprotection.
Proliferating HSPCs are susceptible to chemotherapy damage, and their proliferation is dependent on cyclin-dependent kinase (CDK) 4 and 6 activity in the cell cycle.
When COSELA is given prior to chemotherapy, it transiently arrests HSPCs in the G1 phase of the cell cycle, providing transient inhibition of the CDK4/6 pathway.
This unique effect helps protect HSPCs from damage while chemotherapy targets cancer cells.
After removal of COSELA, HSPCs are able to resume their important role producing blood cells.
The proactive treatment strategy can help protect HSPCs when COSELA is dosed the first time and every time chemotherapy is administered.
COSELA, the first and only myeloprotection therapy for a proactive approach that helps protect HSPCs, the source of blood cell lineages, from damage during chemotherapy.
INDICATION
COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
- COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
WARNINGS AND PRECAUTIONS
Injection-Site Reactions, Including Phlebitis and Thrombophlebitis
- COSELA administration can cause injection-site reactions, including phlebitis and thrombophlebitis, which occurred in 56 (21%) of 272 patients receiving COSELA in clinical trials, including Grade 2 (10%) and Grade 3 (0.4%) adverse reactions. Monitor patients for signs and symptoms of injection-site reactions, including infusion-site pain and erythema during infusion. For mild (Grade 1) to moderate (Grade 2) injection-site reactions, flush line/cannula with at least 20 mL of sterile 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP after end of infusion. For severe (Grade 3) or life-threatening (Grade 4) injection-site reactions, stop infusion and permanently discontinue COSELA. Injection-site reactions led to discontinuation of treatment in 3 (1%) of the 272 patients.
Acute Drug Hypersensitivity Reactions
- COSELA administration can cause acute drug hypersensitivity reactions, which occurred in 16 (6%) of 272 patients receiving COSELA in clinical trials, including Grade 2 reactions (2%). Monitor patients for signs and symptoms of acute drug hypersensitivity reactions. For moderate (Grade 2) acute drug hypersensitivity reactions, stop infusion and hold COSELA until the adverse reaction recovers to Grade ≤1. For severe (Grade 3) or life-threatening (Grade 4) acute drug hypersensitivity reactions, stop infusion and permanently discontinue COSELA.
Interstitial Lung Disease/Pneumonitis
- Severe, life-threatening, or fatal interstitial lung disease (ILD) and/or pneumonitis can occur in patients treated with cyclin-dependent kinases (CDK)4/6 inhibitors, including COSELA, with which it occurred in 1 (0.4%) of 272 patients receiving COSELA in clinical trials. Monitor patients for pulmonary symptoms of ILD/pneumonitis. For recurrent moderate (Grade 2) ILD/pneumonitis, and severe (Grade 3) or life-threatening (Grade 4) ILD/pneumonitis, permanently discontinue COSELA.
Embryo-Fetal Toxicity
- Based on its mechanism of action, COSELA can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should use an effective method of contraception during treatment with COSELA and for at least 3 weeks after the final dose.
ADVERSE REACTIONS
- Serious adverse reactions occurred in 30% of patients receiving COSELA. Serious adverse reactions reported in >3% of patients who received COSELA included respiratory failure, hemorrhage, and thrombosis.
- Fatal adverse reactions were observed in 5% of patients receiving COSELA. Fatal adverse reactions for patients receiving COSELA included pneumonia (2%), respiratory failure (2%), acute respiratory failure (<1%), hemoptysis (<1%), and cerebrovascular accident (<1%).
- Permanent discontinuation due to an adverse reaction occurred in 9% of patients who received COSELA. Adverse reactions leading to permanent discontinuation of any study treatment for patients receiving COSELA included pneumonia (2%), asthenia (2%), injection-site reaction, thrombocytopenia, cerebrovascular accident, ischemic stroke, infusion-related reaction, respiratory failure, and myositis (<1% each).
- Infusion interruptions due to an adverse reaction occurred in 4.1% of patients who received COSELA.
- The most common adverse reactions (≥10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
DRUG INTERACTIONS
- COSELA is an inhibitor of OCT2, MATE1, and MATE-2K. Co-administration of COSELA may increase the concentration or net accumulation of OCT2, MATE1, and MATE-2K substrates in the kidney (e.g., dofetilide, dalfampridine, and cisplatin).
To report suspected adverse reactions, contact Pharmacosmos Therapeutics at 1-800-790-4189 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This information is not comprehensive. Please see full Prescribing Information at COSELA.com.

HEAR FROM A PEER
THE PROTECTIVE MOA OF COSELA
Hear leading medical oncologist Dr. Edward Kim, M.B.A., M.D., share key insights about the novel, proactive, and multilineage mechanism of action (MOA) of COSELA.
NOTE: Dr. Kim is appearing on behalf of Pharmacosmos Therapeutics Inc.
Watch the Video
A NOVEL, PROACTIVE, AND MULTILINEAGE MECHANISM
The mechanism of action of trilacicilib is CDK4/6 inhibition, and it does so transiently.
This effect occurs because it is administered by intravenous infusion prior to chemotherapy, only on each day the chemotherapy is given.
As a result, it protects the blood cell lineages against chemotherapy.
It was an interesting finding in early studies that this transient mechanism was recognized to be clinically relevant.
That’s why the preclinical studies evolved in the manner that they did to show that there was more of a protective mechanism for the hematopoietic stem and progenitor cells.
This is not just another CDK4/6 inhibitor, like the oral therapies. This is a CDK4/6 inhibitor specifically designed to prevent myelosuppression, and proven efficacious in small cell lung cancer patients.
Don’t confuse this with the oral therapies that have a breast cancer indication. Those are for completely different uses. The trilacicilib studies were done to proactively inhibit this pathway to protect hematopoietic stem and progenitor cells.
INDICATION
COSELA® (trilaciclib) is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC).
SELECT IMPORTANT SAFETY INFORMATION
- COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
- Warnings and precautions include injection-site reactions (including phlebitis and thrombophlebitis), acute drug hypersensitivity reactions, interstitial lung disease (pneumonitis), and embryo-fetal toxicity.
- The most common adverse reactions (≥10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
This information is not comprehensive. Please see full Prescribing Information at COSELA.com.

HEAR FROM PEERS
Martin Dietrich, MD, PhD, describes COSELA's unique, proactive approach to help reduce myelosuppression.
QUICK LINKS
RESOURCES FOR Healthcare Professionals