To decrease the incidence of chemotherapy-induced myelosuppression in patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen
For extensive-stage small cell lung cancer (ES-SCLC)
COSELA: DOSED FIRST TIME, EVERY TIME WITH CHEMOTHERAPY
-
The recommended dose of COSELA® (trilaciclib) is 240 mg/m2 per dose. Administered as a 30-minute intravenous (IV) infusion completed within 4 hours of the start of chemotherapy on each day chemotherapy is administered
- Dosage Forms and Strengths. For injection: contains the equivalent of 300 mg of trilaciclib as a sterile, preservative-free, yellow, lyophilized cake in a single-dose vial for reconstitution and further dilution.
SAMPLE INFUSION SCHEDULE
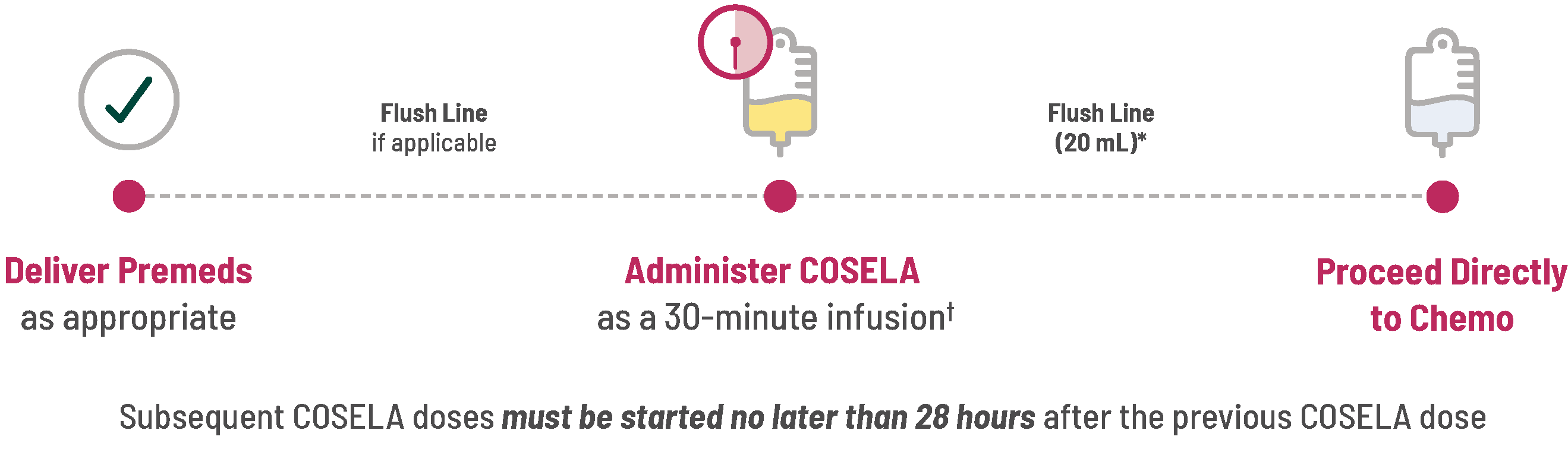
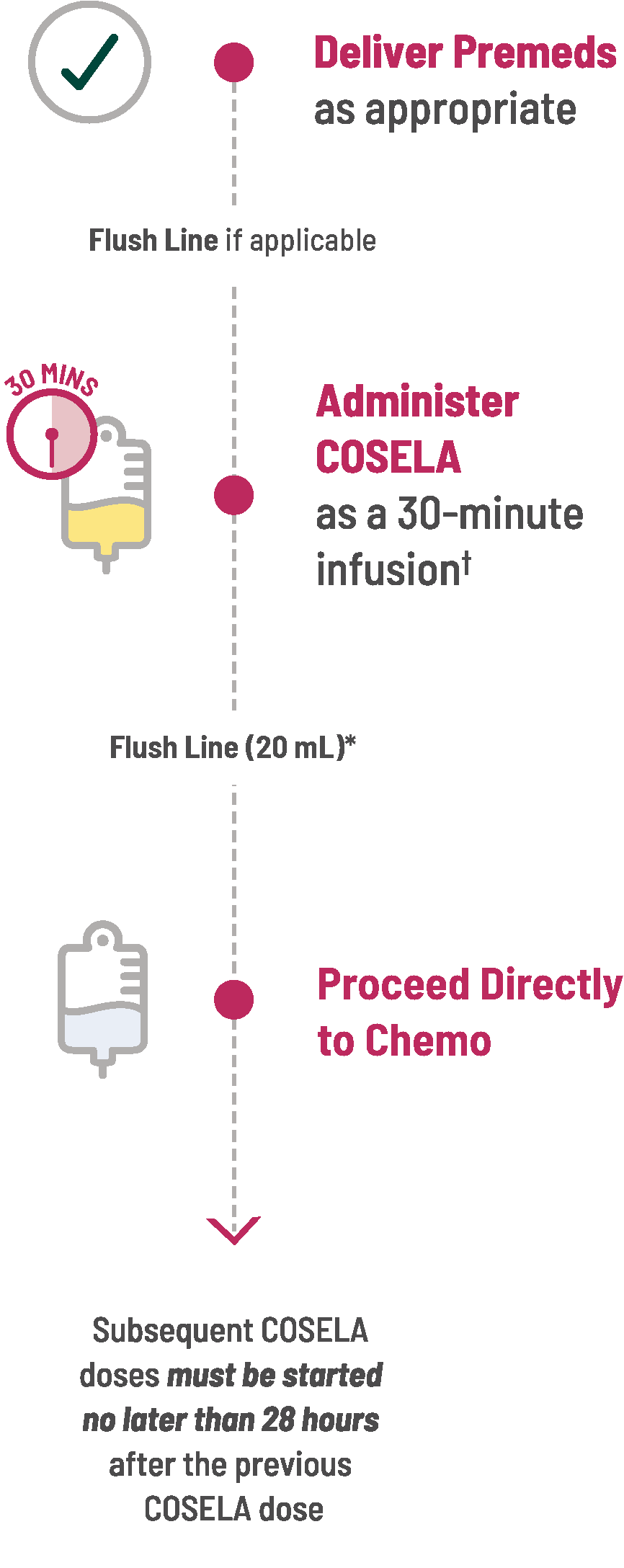
| * | Upon completion of infusion of diluted COSELA solution, the infusion line/cannula MUST be flushed with at least 20 mL sterile 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP. |
| † | COSELA can be given within 4 hours before chemotherapy. |
SEE HOW TO DOSE AND ADMINISTER COSELA
Get a comprehensive overview to administering COSELA. Hear an experienced nurse discuss dosing, preparation, administration, and other infusion-related topics.

CHAPTER: INTRODUCTION TO USING COSELA
Jennifer Webster (00:09):
Hello, I'm Jennifer Webster. And I've been an oncology nurse for more than 30 years.
In this video, you'll be introduced to COSELA. You'll learn about the dosing, preparation, and administration of COSELA, including managing infusion-site reactions and scheduling delays.
COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen, or topotecan-containing regimen, for extensive-stage small cell lung cancer (ES-SCLC).
COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to COSELA.
CHAPTER: INTRODUCING MYELOPROTECTION
Jennifer Webster (00:53):
COSELA helps protect hematopoietic stem and progenitor cells from damage due to chemotherapy and helps protect patients against multiple myelosuppressive consequences.
COSELA is the first and only therapy to proactively protect against chemotherapy-induced myelosuppression.
We call this myeloprotection, a proactive approach to help protect the source of multiple blood cell lineages during chemotherapy.
COSELA is given before chemotherapy. You can help reduce the risk of myelosuppression by administering COSELA as a 30-minute intravenous infusion completed within 4 hours prior to the start of chemotherapy on each day chemotherapy is administered.
CHAPTER: DOSING
Narrator (01:47):
Each single-dose vial for reconstitution contains 300 mg of COSELA as a sterile, preservative-free, yellow, lyophilized cake.
The recommended dose of COSELA is 240 mg/m2 administered as a 30-minute intravenous infusion completed within 4 hours prior to the start of chemotherapy on each day chemotherapy is administered.
This would be 3 consecutive days within a 21-day cycle for etoposide/platinum-containing regimens or 5 consecutive days within a 21-day cycle for topotecan-containing regimens.
The interval between doses of COSELA on sequential days should not be greater than 28 hours.
Jennifer Webster (02:33):
It's important to note that COSELA is a myeloprotection therapy, not a chemotherapy. COSELA, given per recommended dosing, helps protect hematopoietic stem and progenitor cells (HSPCs) from damage due to chemotherapy. Next, you'll learn how COSELA is prepared for administration.
CHAPTER: PREPARING COSELA
Narrator (02:57):
COSELA vials should be stored at controlled room temperature, 68 to 77 degrees Fahrenheit.
COSELA must be reconstituted and then diluted further before giving the IV infusion. Use aseptic technique for reconstitution and dilution.
Be sure to thoroughly inspect the vial and package for any defects or contaminants before administration. This includes checking for things such as particulate matter or discoloration.
Do not use the vial if you suspect any type of contamination.
Reconstitute each 300-mg vial with 19.5 mL of 0.9% Sodium Chloride Injection, or 5% Dextrose Injection, using a sterile syringe to obtain a concentration of 15 mg/mL of COSELA.
Gently swirl the vial contents for up to 3 minutes until it is completely dissolved. Do not shake.
Inspect the reconstituted solution for discoloration and particulate matter.
Narrator (04:03):
Reconstituted COSELA solution should be a clear yellow solution.
Do not use if the reconstituted solution is discolored, cloudy, or contains visible particulates.
If needed, you may store the reconstituted solution in the vial at room temperature for up to 4 hours before transferring it to the infusion bag.
Do not refrigerate or freeze and discard any unused portion after use.
To dilute reconstituted COSELA, withdraw the required volume from the vial of reconstituted COSELA solution and dilute into an IV bag containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection.
Narrator (04:52):
Mix the diluted solution by gentle inversion. Do not shake.
The final concentration of the diluted COSELA solution should be between 0.5 mg/mL, and 3 mg/mL.
Once again, the diluted COSELA in the IV bag should be a clear yellow solution.
Now let's take a look at how bag and diluent choices affect diluted COSELA storage durations.
If using PVC bags, EVA bags, PO bags, or PO/PA IV bags, you may use 5% Dextrose as the diluent. These combinations can be stored up to 12 hours at room temperature.
PVC, EVA, or PO IV bags can be diluted with 0.9% Sodium Chloride—normal saline. These combinations can be stored up to 8 hours at room temperature.
PO/PA IV bags can also be diluted with normal saline. This combination can be stored up to 4 hours at room temperature.
CHAPTER: ADMINISTRATION OF COSELA
Jennifer Webster (06:09):
COSELA patient education resources are available for you to share with patients, so they better understand how COSELA can help them.
The patient education brochure covers topics like how COSELA works and what to expect from therapy.
Here are some important considerations for the administration process.
The recommended dose is 240 mg/m2.
Administer COSELA solution within 4 hours prior to the start of chemotherapy.
Follow your institution’s standard of care for pre-med administration for extensive-stage small cell lung cancer patients.
COSELA should be administered as a 30-minute infusion.
The in-line filter should be a standard 0.2 or 0.22 microns.
Jennifer Webster (06:58):
For example, polyethylene sulfone, polyvinylidene fluoride or cellulose acetate.
Do not administer prepared COSELA solutions with a polytetrafluorethylene, or PTFE, in-line filter.
Do not co-administer other drugs through the same infusion line. Do not co-administer other drugs through a central access device unless the device supports co-administration of incompatible drugs.
Once administration is complete, flush the infusion line with at least 20 mL of sterile 5% Dextrose Injection or 0.9% Sodium Chloride Injection. Flushing the line is a very important final step as flushing may help reduce local irritation or inflammation.
Because infusion-site reactions can occur during the infusion, be sure to monitor the infusion site throughout administration.
CHAPTER: RECOMMENDATIONS FOR ADVERSE REACTIONS, INCLUDING INJECTION-SITE REACTIONS
Jennifer Webster (08:00):
COSELA is not a vesicant. However, some patients may experience injection-site reactions such as redness, tenderness, pain, itching, warmth, or swelling.
Reactions, including phlebitis, an inflammation of the vein, and thrombophlebitis, an inflammation of the vein in conjunction with formation of a blood clot, occurred in 21% of the 272 patients receiving COSELA in clinical trials. 10% were Grade 2 adverse reactions and 0.4% were Grade 3.
Injection-site reactions, including phlebitis and thrombophlebitis, resolved in 49 of 56 patients, or 88%, and led to discontinuation of treatment in 3 of 272 patients, or 1%.
Here are some ways you can address injection-site reactions that may occur.
Jennifer Webster (08:59):
In the case of a Grade 1 adverse reaction, interrupt or slow the infusion and/or change the diluent. For Grade 2 adverse reactions that are severe, stop the infusion and consider rotating the injection site or using central venous access. If 0.9% Sodium Chloride Injection is being used as a diluent and flush, consider changing to 5% Dextrose Injection, as appropriate for subsequent infusions.
Ice or cold packs or warm compresses, depending on the patient's symptoms, may also be considered.
Based on published literature, follow your institution's best practices on managing injection-site irritation or inflammation.
In the event of Grade 3 or 4 adverse reactions, stop the infusion and permanently discontinue COSELA. Permanently discontinue COSELA in cases of acute drug hypersensitivity reactions, Grade 3 or 4, and if Grade 2 recurs, interstitial lung disease or pneumonitis Grades 3 or 4, and if symptomatic Grade 2 recurs, other toxicities Grade 4, and if Grade 3 recurs.
Jennifer Webster (10:14):
Warnings and Precautions for COSELA also include acute drug hypersensitivity, interstitial lung disease or pneumonitis, and embryo-fetal toxicity.
Signs and symptoms to monitor include: acute drug hypersensitivity reactions, including facial, eye and tongue edema, urticaria, pruritus, and anaphylactic reactions, as well as pulmonary symptoms indicative of interstitial lung disease or pneumonitis, such as cough, dyspnea, and hypoxia.
CHAPTER: MANAGING MODIFICATIONS AND SCHEDULING DELAYS
Jennifer Webster (10:48):
It is very important to follow the recommended dosing so that the effect of COSELA is optimized. The recommended dosing is intended to maintain hematopoietic stem and progenitor cells in transient arrest while chemotherapy is present in the body.
If the COSELA dose is missed, discontinue chemotherapy on the day the COSELA dose was missed. Consider resuming both COSELA and chemotherapy on the next scheduled day for chemotherapy. If COSELA is discontinued, wait 96 hours from the last dose of COSELA before resuming chemotherapy only.
Jennifer Webster (11:30):
Thank you for watching.
If you'd like additional information or have questions, visit COSELA.com/request-information to access a G1 Clinical Nurse Educator.
You can also find more information about COSELA at COSELA.com and in the Full Prescribing Information at COSELA.com.
CHAPTER: INDICATION AND IMPORTANT SAFETY INFORMATION
Narrator (11:54):
INDICATION
COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
- COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
WARNINGS AND PRECAUTIONS
Injection-Site Reactions, Including Phlebitis and Thrombophlebitis
- COSELA administration can cause injection-site reactions, including phlebitis and thrombophlebitis, which occurred in 56 (21%) of 272 patients receiving COSELA in clinical trials, including Grade 2 (10%) and Grade 3 (0.4%) adverse reactions. Monitor patients for signs and symptoms of injection-site reactions, including infusion-site pain and erythema during infusion. For mild (Grade 1) to moderate (Grade 2) injection-site reactions, flush line/cannula with at least 20 mL of sterile 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP after end of infusion. For severe (Grade 3) or life-threatening (Grade 4) injection-site reactions, stop infusion and permanently discontinue COSELA. Injection-site reactions led to discontinuation of treatment in 3 (1%) of the 272 patients.
Acute Drug Hypersensitivity Reactions
- COSELA administration can cause acute drug hypersensitivity reactions, which occurred in 16 (6%) of 272 patients receiving COSELA in clinical trials, including Grade 2 reactions (2%). Monitor patients for signs and symptoms of acute drug hypersensitivity reactions. For moderate (Grade 2) acute drug hypersensitivity reactions, stop infusion and hold COSELA until the adverse reaction recovers to Grade ≤1. For severe (Grade 3) or life-threatening (Grade 4) acute drug hypersensitivity reactions, stop infusion and permanently discontinue COSELA.
Interstitial Lung Disease/Pneumonitis
- Severe, life-threatening, or fatal interstitial lung disease (ILD) and/or pneumonitis can occur in patients treated with cyclin-dependent kinases (CDK)4/6 inhibitors, including COSELA, with which it occurred in 1 (0.4%) of 272 patients receiving COSELA in clinical trials. Monitor patients for pulmonary symptoms of ILD/pneumonitis. For recurrent moderate (Grade 2) ILD/pneumonitis, and severe (Grade 3) or life-threatening (Grade 4) ILD/pneumonitis, permanently discontinue COSELA.
Embryo-Fetal Toxicity
- Based on its mechanism of action, COSELA can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should use an effective method of contraception during treatment with COSELA and for at least 3 weeks after the final dose.
ADVERSE REACTIONS
- Serious adverse reactions occurred in 30% of patients receiving COSELA. Serious adverse reactions reported in >3% of patients who received COSELA included respiratory failure, hemorrhage, and thrombosis.
- Fatal adverse reactions were observed in 5% of patients receiving COSELA. Fatal adverse reactions for patients receiving COSELA included pneumonia (2%), respiratory failure (2%), acute respiratory failure (<1%), hemoptysis (<1%), and cerebrovascular accident (<1%).
- Permanent discontinuation due to an adverse reaction occurred in 9% of patients who received COSELA. Adverse reactions leading to permanent discontinuation of any study treatment for patients receiving COSELA included pneumonia (2%), asthenia (2%), injection-site reaction, thrombocytopenia, cerebrovascular accident, ischemic stroke, infusion-related reaction, respiratory failure, and myositis (<1% each).
- Infusion interruptions due to an adverse reaction occurred in 4.1% of patients who received COSELA.
- The most common adverse reactions (≥10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
DRUG INTERACTIONS
- COSELA is an inhibitor of OCT2, MATE1, and MATE-2K. Co-administration of COSELA may increase the concentration or net accumulation of OCT2, MATE1, and MATE-2K substrates in the kidney (e.g., dofetilide, dalfampridine, and cisplatin).
To report suspected adverse reactions, contact Pharmacosmos Therapeutics at 1-800-790-4189 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This information is not comprehensive. Please see full Prescribing Information at COSELA.com.

HEAR HOW ONE PRACTICE STARTED USING COSELA



INDICATION
COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide- containing regimen or topotecan-containing regimen for ES-SCLC.
Monica:
Incorporating COSELA into our practice was very smooth.
It only adds about 30 minutes of chair time, and it's such a great experience for the patients because they feel more confident in receiving the chemotherapy because we've already previously discussed with the patient why we're doing it, and that it's really there to help protect them so that they can continue with their treatment goals.
So the process of including COSELA in our practice was simply adding it to the order set so that when the regimen is ordered, it just automatically populates each time we add that specific regimen.
We now have it to where the infusion nurses are accustomed to giving this medication along with their chemotherapy regimen, and they'll even say, "I see that you're giving this regimen, but I don't see COSELA listed."
So they'll even know to come and ask me or the oncologist, "I don't see COSELA. Is this something that you want to add?" So, that way they act as a double-checking system.
Important Safety Information
- COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
- Warnings and precautions include injection-site reactions (including phlebitis and thrombophlebitis), acute drug hypersensitivity reactions, interstitial lung disease (pneumonitis), and embryo-fetal toxicity.
- The most common adverse reactions (≥10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
- This information is not comprehensive. Please see the full Prescribing Information at COSELA.com

Recommended actions for ADVERSE REACTIONS
- Upon completion of infusion, flush line/cannula with at least 20 mL sterile 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP
- If 0.9% Sodium Chloride Injection, USP is being used as a diluent/flush, consider changing to 5% Dextrose Injection, USP as appropriate for subsequent infusions
Note that COSELA is not a vesicant
Refer to the Prescribing Information for additional details.
Additional considerations: Ice/cold packs or warm compresses, depending on the patient’s symptoms, may be considered per institutional guidelines.1,2
Injection-Site Reaction Incidence
Injection-site reactions, including phlebitis and thrombophlebitis, occurred in 56 (21%) of the 272 patients receiving COSELA in clinical trials. Occurrence of Grade 2 adverse reactions was 10% and Grade 3 adverse reactions was 0.4%.
- Injection-site reactions, including phlebitis and thrombophlebitis, resolved in 49 (88%) of the 56 patients and led to discontinuation of treatment in 3 (1%) of the 272 patients
ADDITIONAL WARNINGS AND PRECAUTIONS
Warnings and precautions for COSELA also include acute drug hypersensitivity, interstitial lung disease (ILD)/pneumonitis, and embryo-fetal toxicity.
Signs and Symptoms to Monitor
- Acute drug hypersensitivity reactions: acute drug hypersensitivity reactions, including facial, eye, and tongue edema, urticaria, pruritus, and anaphylactic reactions
- ILD/pneumonitis: pulmonary symptoms indicative of ILD/pneumonitis such as cough, dyspnea, and hypoxia
| * | If 0.9% Sodium Chloride Injection, USP is being used as a diluent/flush, consider changing to 5% Dextrose Injection, USP as appropriate for subsequent infusions. |
| † | Stop infusion in extremity and rotate site of infusion to site in alternative extremity. |
| ‡ | Defined as: Grade 2=Moderate; minimal, local, or noninvasive intervention indicated; limiting Activities of Daily Living (ADL). Grade 3=Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL. Grade 4=Life-threatening consequences; urgent intervention indicated. |
| § | Defined as: Grade 2=Symptomatic. Grade 3=Severe symptoms; limiting self-care ADL; oxygen indicated. Grade 4=Life-threatening respiratory compromise; urgent intervention indicated (e.g., tracheotomy or intubation). |
| ‖ | Defined as: Grade 3=Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL. Grade 4=Life-threatening consequences; urgent intervention indicated. |
Unexpected THERAPY INTERRUPTION
The interval between doses of COSELA on sequential days should not be greater than 28 hours.
Missed Treatment Session(s)
- If the COSELA dose is missed, discontinue chemotherapy on the day the COSELA dose was missed. Consider resuming both COSELA and chemotherapy on the next scheduled day for chemotherapy
Discontinuation of Treatment
- If COSELA is discontinued, wait 96 hours from the last dose of COSELA before resumption of chemotherapy only
INDICATION: COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC).

Dosing & Administration Guide
Review Pivotal Study
SAFETY
QUICK LINKS

